 |
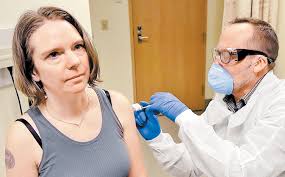
| Coronavirus vaccine first trial test administrated by Kaiser Permanente Washington Health Institute of Research in Seattle USA |
Responding to pandemic coronavirus disease, US researcher
gave first injection in human body to test coronavirus vaccine safety on last
Monday.
We are not sure that coronavirus vaccine trial version is
worthy; we apply its doses on many other volunteers to find out its safety. The
person who is spotted for test on trial basis is a 43 year lady, working as
operation manager at a small tech company. The lady was vaccinated in an exam
room, at Kaiser Permanente Washington Health institute of Research in Seattle.
“We all feel so helpless. This is an amazing opportunity for
me to do something,” Jennifer Haller, 43, mother of two teenage daughter told
before taking vaccine.
“I’m feeling great.” She said with big smile after leaving
the exam room while being vaccinated.
The second volunteer name is Neal Browning, a Microsoft
network engineer. He is 46 years old belongs to Bothell, Washington. “Her
daughters feel proud on him as Volunteer,” he said.
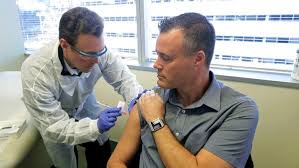 |
| Neal Browning, 46, Bothell, Washington-a Microsoft network Engineer-A volunteer Fighter against Coronnvirus. |
The major goal of this first set of tests is to determine if
the vaccine is safe. If it is, later study will find out how efficient it is.
“We’re team coronavirus now,” said Dr Lisa Jackson study
leader at Kaiser Permanente on the eve of the experiment. “Everyone wants to do
what they can in this emergency.”
The Trial was “launched in record speed”, Dr. Anthony Fauci,
the institute’s Director, said in a statement. Further, President Donald Trump
praised on the rapid development on vaccine after 65 days when china had shared
the virus’ genetic sequence.
The production of a potential vaccine in such a short span
of time is unprecedented. The miracle
was possible because the scientist already conducting studies on corona related
viruses that cause diseases like SARS and MERS.
If the coronavirus vaccine proves safe, it still not
available for use on large scale for, at least one year is needed to make it
available to everyone.
The coronavirus vaccine used to conduct test at Kaiser Permanente
Washington Health Institute of Research, is made by Moderna Inc in
collaboration with National Institute of Allergy and Infectious diseases.
The genetic material used to produce this vaccine is termed
as RNA-1273. Participants have no fear
to be harmed or infected with vaccine, because coronavirus is not part of it. The
genetic material RNA can develop vaccine quickly, said Dr. Barney Graham, the Deputy
Director of the institute’s Vaccine Research Center. Other companies are
applying different approaches to develop the vaccine against the coronavirus outbreaks.
This vaccine is not the last hope, many other research
groups are in competition to produce vaccine or medicine that defeat this WHO
announced pandemic disease, known as Covid-19 or Coronavirus outbreaks. Another
player, known as Inovio Pharmaceuticals, has plan to starts its own safety
research on coronavirus vaccine from next month in the South Korea, China and
USA.
Picking Seattle city for testing anti-coronavirus vaccine is
not the known cases but the WHO announcement declaring it pandemic due to rapid
global spread. Further, The Seattle Research Institute is administrated by
Government that uses to test all types of vaccines.
The Covid-19 has severely shattered the world’s social and
economic aspects of life since its discovery from China in January. The
pandemic disease coronavirus has hit the Italy hardly. Countries from all over
the world are taking measures such as restricting travelers, suspending flights
operations, cancelling entertainment and sports events, social distancing, closing schools, and so on
to control and overcome the impact of it.
Initially, 45 healthy volunteers have been enrolled for
conducting trial test, aging from 18 to 55 years. Four volunteers are tested on
Monday and Four on Tuesday, and then a pause is being given for 15 days, total
three different doses will be tested, to check the side effects of the vaccine
or it helps improving immune system of the humans, and looking for signals that
sets further direction for the coronavirus
vaccine progress in more better way.
The coronavirus vaccinated volunteers will be under review for
years, but the reports and data will be available few weeks after the vaccine
injected, said Stephane Bancel, the chief executive at Moderna.
Mostly studies have been conducting across the globe to
target the protein aptly named “spike” prevailing on the surface of the
coronavirus that help it enter into the human immune system. Block
protein intrusion to safe people from being infected.

nice content keep it up
ReplyDelete